Coronavirus
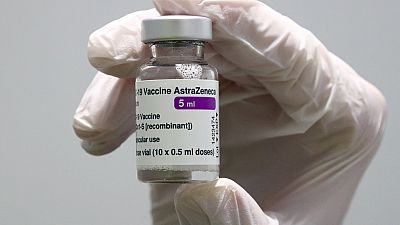
Pharmaceutical** giant AstraZeneca has formally asked the European Medicines Agency to withdraw its COVID-19 vaccine authorization.**
In an update on the European Medicines Agency's website Wednesday, the regulator said that the approval for AstraZeneca's Vaxzevria had been withdrawn “at the request of the marketing authorization holder.”
The vaccine, initially approved in January 2021, faced safety concerns over rare blood clots, prompting various countries to halt its use temporarily.
While the EU regulator determined that the overall risk was low, doubts lingered. Additionally, limited data on its effectiveness in older adults led to initial restrictions.
READ ALSO:Hopes of HIV cure after breakthrough using gene-editing 'scissors'
Billions of doses of the AstraZeneca vaccine were distributed to poorer countries through a U.N.-coordinated program, as it was cheaper and easier to produce and distribute. However? studies later suggested that the pricier messenger RNA vaccines made by Pfizer-BioNTech and Moderna provided better protection against COVID-19 and its many variants, and most countries switched to those shots.
The U.K.'s national coronavirus immunization program in 2021 heavily relied on AstraZeneca's vaccine, which was largely developed by scientists at Oxford University with significant financial government support.
But even Britain later resorted to buying the mRNA vaccines for its COVID booster vaccination programs and the AstraZeneca vaccine is now rarely used globally.












01:05
Study finds millions of children at risk as global vaccine rates fall
Go to video
What to know about the COVID variant that may cause 'razor blade' sore throats
01:29
US medication safety agency approves biannual preventive HIV shot
01:02
As cholera cases surge, African leaders urge local production of vaccine
Go to video
England to launch world-first Gonorrhoea vaccination programme
Go to video
WHO approves landmark pandemic agreement to improve response in event of future pandemic