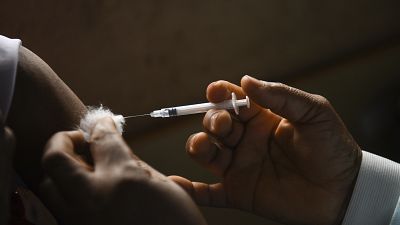
AstraZeneca
A World Health Organization body of experts on immunization said on Wednesday the COVID-19 AstraZeneca vaccine was safe, a day after German health officials agreed to restrict its use in people under the age of 60, amid fresh concerns over unusual blood clots reported in a tiny number of those who received the shots.
WHO's Director of the Department of Immunization, Vaccines and Biologicals Dr. Kate O'Brien said that it was "clear" that "the benefit-risk assessment for the AstraZeneca vaccine, given the range of evidence on both efficacy, effectiveness, safety and quality manufacturing" still "weighs very heavily in favor of the use of the vaccine."
"It is a safe vaccine," she underlined.
O'Brien made the remarks as the WHO's Strategic Advisory Group of Experts (SAGE) on Immunization was presenting findings from its regular meeting with a focus on the status of vaccines for COVID-19, Ebola and other diseases.
Dr. Alejandro Cravioto, who is the head of a WHO panel of immunization experts, said two Chinese vaccines from Sinovac and Sinopharm, which the UN's health agency is assessing, have so far demonstrated "levels of efficacy that would be compatible with the requirements of WHO."
Speaking at a briefing with journalists on Wednesday he said those levels would be at least 50% effective and "preferably close to or above 70%."
Craviato noted that many national regulators have already approved the two vaccines for use, even without an emergency use listing that the Chinese manufacturers are seeking from WHO.
Such a listing would allow them to be included in the UN-backed global vaccine rollout program known as COVAX.
A WHO decision on any emergency use listing for the two Chinese vaccines could come at the earliest next month, the agency says, but it has not provided specifics.
Experts probing links between the AstraZeneca coronavirus vaccine and blood clots have found no specific risk factors, including age, but are carrying out further analysis, the EU's drug regulator said Wednesday.
The European Medicines Agency (EMA) said however that its safety committee expected to issue an "updated recommendation" on the controversial vaccine after its monthly meeting next week.
Germany on Tuesday became the latest in a series of countries to advise against using the AstraZeneca jab for younger people after rare reports of clotting, despite the EMA saying it is safe.












01:04
South Africa reports new bird flu outbreak on poultry farms
Go to video
Over 40 killed in attack on Sudanese hospital: WHO Chief condemns “Appalling” strike
Go to video
Ghana confirms 34 new Mpox cases, total rises to 79
01:07
WHO says the mpox outbreak remains a public health concern
01:34
Flavored tobacco products luring youth to addiction, death - WHO
01:00
New cholera outbreak in Sudan kills 172 people in a week