USA
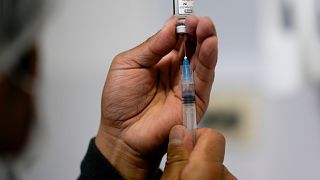
The FDA approved updated COVID-19 vaccines from Pfizer, Moderna, and Novavax Wednesday, but narrowed their use for millions of Americans. The shots are now only recommended for seniors or people with at least one high-risk condition, such as asthma or obesity.
Pfizer’s vaccine will no longer be available for children under 5, while Moderna’s mRNA vaccine retains approval for children as young as 6 months—but only for those with serious health conditions. Novavax’s protein-based shot is restricted to people 12 and older under the same criteria.
The new vaccines target a recent coronavirus subtype, LP.8.1, and are set to start shipping immediately. However, access may be delayed as distribution depends on health insurers, pharmacies, doctors, and state authorities. Healthy adults and children who want a shot may face additional barriers, including potential out-of-pocket costs exceeding $150.
Health officials say the move reflects reduced risk for the general population, as most Americans already have immunity from previous vaccinations or infections. Critics, including the American Academy of Pediatrics, warn that the limits may block families from protecting children.
COVID-19 remains a serious threat for seniors and people with underlying conditions, with preliminary CDC data showing nearly 47,500 deaths in the U.S. last year. The FDA also revoked several other emergency-use therapies from the pandemic era, including convalescent plasma.
The new guidance marks a major shift from previous policy, which recommended annual COVID-19 shots for all Americans six months and older.










01:04
Rising anger in Africa over 'lopsided' US health funding agreements
01:32
Legal case sheds light on US-Nigeria tensions over religious freedom
01:08
Zambia rejects U.S. health aid over mining partnership ties
01:00
Pix of the Day, 25 February 2026
01:48
Iran warns US: choose 'table of diplomacy' or face 'firm blow'
01:00
Iran faces renewed student protests in Tehran as new semester begins