Malaria
Loss of life, loss of livlihoods and homelessness have already afflicted these flood marooned refugees in Pakistan.
Now these living conditions means they also face sickness and and without protection malaria is a major threat.
Health agencies try to protect people against infection with sprays, nets and a vaccine for children, there are also preventative medicines.
But despite all these, there were 241 million cases of malaria in 2020 and an estimated 627,000 deaths according to the latest malaria report from the World Health Organization (WHO)
The WHO says these strategies, which are also used for other mosquito borne diseases such as Zika and dengue, are only partially effective.
In the meantime the insects are becoming inceasingly resistant to insecticides.
Scientists have for many years been investigating whether they can control the life cycle of mosquitoes by manipulating their DNA, thereby creating genetically modified mosquitoes.
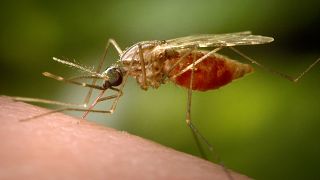
The disease is spread by a parasite Plasmodium falciparum which grows and reaches maturation inside the female Anopheles mosquito.
It is spread when someone is bitten by an infected mosquito.
Climate is key according to the CDC, it says Plasmodium falciparum can't complete its growth cycle if temperatures are below 20 degrees Celcius.
Scientists are looking at different ways to prevent the parasite from reaching maturiity and becoming infectious.
As well as deploying nets sprayed with insecticide, Tanzanian health officials called in researchers in 2019 to deploy drones to spray a silicone-based liquid on large expanses of stagnant water in rice paddies.
They are a well known breeding ground for malaria-carrying mosquitoes to lay their eggs.
The silicone substance is spread across the water to prevent the eggs from hatching.
In laboratories around the world researchers are investigating whether there are genetic solutions.
Researchers at Imperial College in London have also been investigating how to alter the mosquitoes genetically to stunt the development of the parasite as well as shortening the life of the mosquito.
Professor George Christophides explains how they aim to reduce infection.
He says: "We prolonged the time that the parasite needs to be inside the mosquito in order to be transmitted."
Christophides says it's important to understand the life cycle of the parasite as well as the mosquito.
"The important thing that we have to understand is that the mosquito is not just a mere carrier of the parasite it doesn't take it from one human infected and carries it to another one. The parasite needs to spend time in the mosquito and it has to develop is like development of an organism. It has to develop from initially gametes like a sperm and an egg into a zygote like the embryo, and then becoming a (blasto)cyst then in this cyst, we have some new parasites forming that will infect the salivary glands of the mosquitoes so that when it bites again, these parasites in the salivary glands will be injected into the new person. And this whole, this period, this developmental time takes about in nature about 10 to 12 days so what we did here is to prolong that, make it longer. So instead of 10 to 12 days, it will be like 15 to 17 days that will take for the parasite to become infectious for another human inside the mosquito."
By increasing the essential development time the parasite needs to become fully functioning the scientists cut down the window for potential trasmission because the life of the mosquito is short.
However their DNA changes also shorten the life of the actual mosquito.
"Now, why is this important? Because mosquitoes in nature usually live short. They have a short life, and the average mosquito in nature will live about 7 to 10 days. So he doesn't have enough time to transmit the parasite. So most mosquitoes will never have the chance to transmit the parasite. It's only 10% of the mosquitoes out there that live long enough to be able to transmit the parasite. By prolonging the life, the developmental time that the parasite needs inside the mosquito to become infectious, it means that this 10% becomes now much smaller. It's only a very tiny fraction of mosquitoes that can live long enough to transmit the parasite. The other thing, at the same time that we managed to do is that we cut the mosquito's life a bit short. So instead of living 7 to 10 days on average it will live a bit shorter. So the two things combined together now can lead to blocking malaria transmission in the field. So this is the innovation," says Christophides.
The Imperial Team are planning further laboratory trials in Rwanda, producing blood meals for the GM insects from the blood of patients infected by malaria.the int
They are also planning field trials of the mosquitoes with the modified DNA, however this part is far more tricky.
But it's not possible to just release these mosquitoes into wild infested areas hoping they will multiply, they're unlikely to survive.
This is because of its genetic changes the mosquito has been weakened, so scientists need to make futher changes to increase their survival.
To accomplish this they create what they describe as a "gene drive".
According to the Proceedings of the National Academy of Sciences (PNAS) this is a type of genetic engineering which favours specific heriditary characteristics to increase the likelihood that these specific characteristics are quickly spread through the population and passed onto the next generation.
It's this sort of introduced natural selection which sounds alarm bells among people who are concerned that GM mosquitoes could have an impact on the natural environment of which we are still unaware.
"These modifications that we did makes them weaker because they live shorter. So it will be eliminated naturally by the natural selection after a few generations they will be eliminated. So there is no way to spread this modification in the field unless you combine it with what we call the "gene drive", which is not what we are. We have tested it here. But we have not really put in this full implementation. So what you do is you combine this genetic modification with a driver, what we call a driver, which will take this modification and spread it quickly through the populations. Now, the details of how this is done doesn't matter, but essentially it drives it through. So within a few generations, all the mosquitoes in the field, in an area where you release, they will become genetically modified. This is the thing that scares more the people at the moment, the gene drive."
BioNTech the first company to create an mRNA vaccine for COVID-19 says it's developing its first mRNA vaccine for the prevention of malaria for the African continent.
The Imperial team says it's important that science keeps pushing the boundaries to overcome diseases like malaria.











02:17
London Mayor Sadiq Khan visits Lagos to strengthen UK-Nigeria tech and creative ties
02:03
Muhammadu Buhari's legacy: higlight of his presidential tenure
01:01
Kenya: Visa-free travel now available for many African and Caribbean countries
02:02
Could AI help fight mosquito-borne diseases?
Go to video
First Malaria treatment for babies approved
01:09
Liverpool star Jota has died in a car accident - Spanish police