Namibia
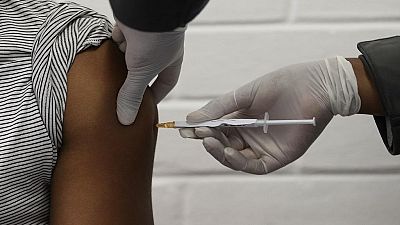
Namibia has appointed a technical team to look into logistical requirements of importing a COVID-19 vaccine.
The southern African country’s minister of health said the team was instructed to study the storage, transport and distribution needs, local newspaper The Namibian reported on Friday.
Namibia lacks the infrastructure needed to store or distribute a COVID-19 vaccine. Most of the vaccine candidates so far require ultra-cold conditions for storage and distribution.
Namibia has paid $1.9m to the COVAX programme, a global initiative aimed at working with vaccine manufacturers to ensure equitable access to safe and effective vaccines - to secure the medicines for her people.
The country targets to vaccinate 20% of its population. Frontline health workers and people of advanced age will be the first recipients of the jabs.
Namibia has recorded 16,097 cumulative cases, 14,332 recoveries and 160 deaths.
The country has a population of nearly 2.5 million people.
Neighboring Angola on Thursday said it expected to receive five million doses of a COVID-19 vaccine in February 2021.
Health Minister Silvia Lutucuta said seven million more doses would be delivered in April in partnership with COVAX.
Angola has so far reported 15,925 positive cases, 362 deaths, and 8,679 recoveries.
Egypt on Thursday took delivery of the first batch of China’s Sinopharm vaccine.
Morocco on Wednesday announced that it was gearing up for an ambitious COVID-19 vaccination program, aiming to vaccinate 80% of its adults in an operation starting this month.
The North African kingdom is pinning its hopes on two vaccine candidates, one developed by China’s Sinopharm and the other by Britain’s Oxford University and AstraZeneca.
It seeks to vaccinate 80% of its adults, or 25 million people, as soon as the vaccines get regulatory approval.










Go to video
What to know about the COVID variant that may cause 'razor blade' sore throats
11:16
Angola hosts U.S.-Africa summit amid calls to revive trade ties {Business Africa}
Go to video
WHO approves landmark pandemic agreement to improve response in event of future pandemic
Go to video
Gabon: former president Ali Bongo and family go into exile in Angola
Go to video
Cholera outbreak in Angola kills nearly 600 people
Go to video
Nigeria completes $3.4 Billion IMF COVID-19 loan repayment, faces ongoing annual charges